Draw the structures of optical isomers of:
(i) [Cr(C2O4)3]3– (ii) [PtCl2(en)2]2+
(iii) [Cr(NH3)2Cl2(en)] +
Optical isomers are the one which rotate the plane polarized light by a certain angle when passed through them and are mirror images of each other, also called as stereoisomer.
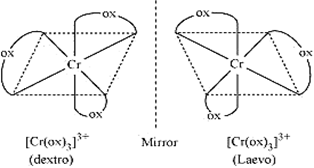
(i) C2O42- is a bidentate ligand so it can attach to central atom at two sites, forming a chelate ring. In the complexes of this type, three symmetrical bidentate chelating ligands AA are coordinated to the central metal atom M. Such complexes do not possess any element of symmetry and are optically active. Moreover, these complexes can be resolved into optical isomers.
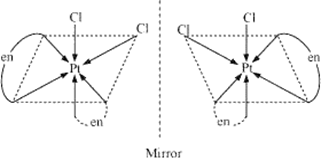
(ii) In this complex Cl is an unidentate ligand with one site of attachment whereas -en(ethylenediamine) is bidentate ligand with two sites of attachment. The complexes in which two symmetrical bidentate chelating ligands AA and two monodentate ligands a, are coordinated to central metal atom M, exhibit the phenomenon of optical isomerism and can be resolved into their optical isomers.
An example of this type of complexes is given as shows both geometrical as well as optical isomerism. Its cis form is unsymmetrical, while the trans form is symmetrical because it contains a plane of symmetry. Hence, optical isomerism is shown by cis form.
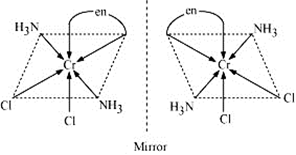
(iii) In this there are three types of ligands. One is ammonia which is neutral (nitrogen donates the lone pair of electron to metal), Cl - ligand is unidentate and -en is bidentate ligand. Coordination number is 6. Two optical isomers are possible due to presence of 3 types of ligands.