Draw all the isomers (geometrical and optical) of:
(i) [CoCl2(en)2] +
(ii) [Co(NH3)Cl(en)2]2+
(iii) [Co(NH3)2Cl2(en)] +
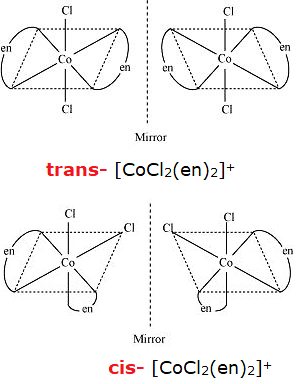
(i) [CoCl2(en)2] +
Two types of ligand : chloride ion is unidentate ligand and -en(ethylenediamine) is bidentate ligand with two sites of attachment. The complexes in which two symmetrical bidentate chelating ligands AA and two monodentate ligands a, are coordinated to central metal atom M, exhibit the phenomenon of optical isomerism and can be resolved into their optical isomers.
An example of this type of complexes is given as shows both geometrical as well as optical isomerism. Its cis form is unsymmetrical, while the trans form is symmetrical because it contains a plane of symmetry. Hence, optical isomerism is shown by cis form.
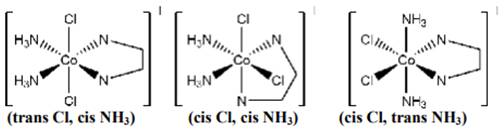
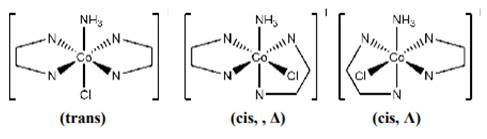
(ii) [Co(NH3)Cl(en)2]2+
In this there are three types of ligands. One is ammonia which is neutral (nitrogen donates the lone pair of electron to metal), Cl - ligand is unidentate and -en is bidentate ligand.
Geometrical isomers are possible because there is no plane of symmetry, so cis and trans geometrical isomers exists. Trans isomer will not show optical isomerism as the mirror image formed is superimposible of each other instead cis isomer show optical isomerism.
(iii) In this complex there are three types of ligands. There is no axis of symmetry that can divide the whole complex in exactly two equal halves, so there exists geometrical isomers.