Discuss the nature of bonding in the following coordination entities on the basis of valence bond theory:
(i) [Fe(CN)6]4– (ii) [FeF6]3– (iii) [Co(C2O4)3]3– (iv) [CoF6]3–
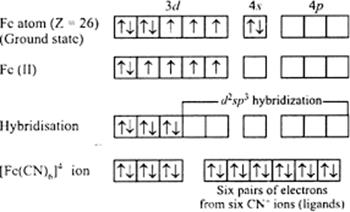
(i) In the coordination entity iron exists in + 2 oxidation state.
Overall charge balance:
X + 6(-1) = -4
X = + 2.
Its electronic configuration is: 3d6
CN- is strong field ligand so it causes pairing of the unpaired electron and undergoes hybridisation to form 6 d2sp3 hybrid orbitals to be filled by the six cyanide ions. It's geometry is octahedral with no unpaired electrons and hence is diamagnetic complex.
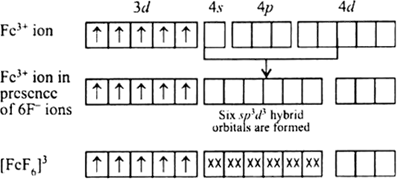
(ii) In the coordination entity iron exists in + 3 oxidation state.
Overall charge balance:
X + 6(-1) = -3
X = + 3.
Its electronic configuration is: 3d6
F- is weak field ligand so it not causes pairing of the unpaired electron and undergoes hybridisation to form 6 sp3d2 hybrid orbitals to be filled by the six fluoride ions. It's geometry is octahedral and is paramagnetic.
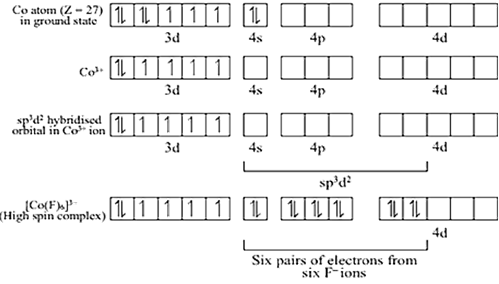
(iii) In the coordination entity cobalt exists in + 3 oxidation state.
Overall charge balance:
X + 3(-2) = -3
X = + 3.
Its electronic configuration is: 3d5
C2O4- is weak-field ligand so it not causes pairing of the unpaired electron and undergoes hybridisation to form 6 sp3d2 hybrid orbitals to be filled by the three oxolate ions(it is bidentate ligand). It's geometry is octahedral with unpaired electrons and hence is paramagnetic complex.
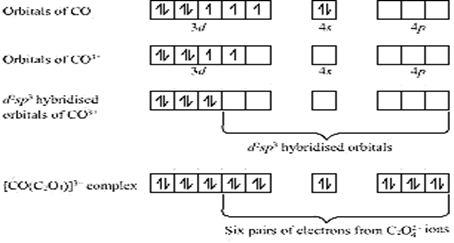
(iv) In the coordination entity cobalt exists in + 3 oxidation state.
Overall charge balance:
X + 6(-1) = -3
X = + 3.
Its electronic configuration is: 3d5
Fluoride ion is weak field ligand so it not causes pairing of the unpaired electron and undergoes hybridisation to form 6 sp3d2 hybrid orbitals to be filled by the six fluoride ions. It's geometry is octahedral with unpaired electrons and hence is paramagnetic complex.