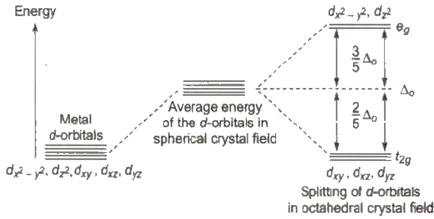
Draw figure to show the splitting of d orbitals in an octahedral crystal field.
In octahedral complex the splitting of the d orbital will be such a way that the dx2-y2 and dz2 orbitals which face towards the axes along the direction of the ligand will experience more repulsion and will be raised in the energy and the other three orbitals which are directed between the axes are lowered in energy.
21