Discuss the nature of bonding in metal carbonyls.
Compounds containing carbonyl
ligands only are known as homoleptic carbonyl. Such types of compounds are formed by most of the transition metals. These metal carbonyls always have simple, well-defined structures. In metal carbonyls the metal - carbonyl bond possess both s and p.character. M-C-bond is sigma bond. It is formed by the donation of lone pair of electrons of the carbonyl carbon into the vacant orbital of the metal. The M-C pi bond is formed by the donation of a pair of electron from a filled d orbital of a metal into the vacant antibonding π orbital of carbon monoxide. Such type of metal to ligand bonding creates a synergic effect which strengthens the bond between CO and metal.
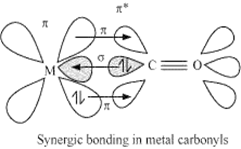
25