Write down the IUPAC name for each of the following complexes and indicate the oxidation state, electronic configuration and coordination number. Also give stereochemistry and magnetic moment of the complex:
(i) K[Cr(H2O)2(C2O4)2].3H2O (iii) [CrCl3(py)3] (v) K4[Mn(CN)6]
(ii) [Co(NH3)5Cl-]Cl2 (iv) Cs[FeCl4]
(i) The complex is an anion with chromium as central atom, 2 water molecules and 2 oxolate ions with -2 negative charge each. Balance overall charge as 0, we get oxidation state of Cr as:
X + 2(0) + 2(-2) = -1
X = + 3.
Name of compound: potassium diaquadioxolatochromate(III) trihydrate.
Electronic configuration of Cr: 3d3, t2g3
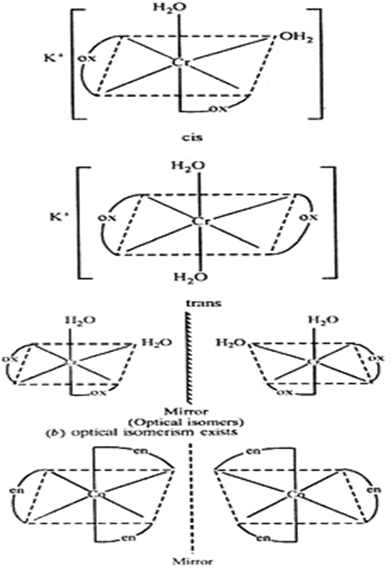
Co-ordination no. Of complex: 6 (as oxolate ion is bidentate ligand)
As both the water molecule and oxolate ion are weak field ligand, so they do not cause pairing up of electron.
μ = [n(n + 2)]1/2
μ = [3×5]1/2
μ = 4BM.
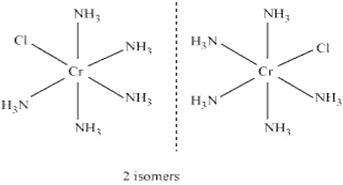
(ii) The complex is a cation with cobalt as central atom, five neutral ammonia molecules and one chloride ion. Balance overall charge as 0, we get,
X + 5(0) + 1(-1) = + 2
X = + 3.
Oxidation state of cobalt is + 3.
Name of compound is pentaamminechloridocobalt(III) chloride.
Coordination no. is 6.
Electronic configuration of cobalt: 3d6
As ammonia is a strong field ligand so it causes pairing of the electron. No. Of unpaired electrons:0
μ = [n(n + 2)]1/2
μ = [0]1/2
μ = 0BM.
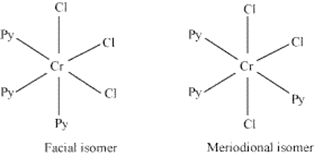
(iii) The complex is neutral with chromium as central atom, three chloride ions and 3 pyridine molecules which are neutral.
Name of compound is trichloridotripyridinechromium(III).
Balance overall charge as 0.
X + 3(-1) + 3(0) = 0
X = + 3.
Oxidation state of chromium is + 3.
Electronic configuration:3d3
Coordination no. Is 6.
As chloride ion and pyridine molecule botha re weak field ligand so they do not cause pairing of electron.
μ = [n(n + 2)]1/2
μ = [3×5]1/2
μ = 4BM
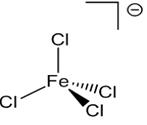
(iv) The complex is anion with iron as central atom, and 4 chloride ions. Balance overall charge:
X + 4(-1) = -1
X = + 3
Oxidation state of Fe: + 3.
Electronic configuration:3d6
Name of compound: Caesium tetrachloridoferrate(III).
Coordination no. is 4.
As chloride ion is weak field ligand ,it does not cause pairing of electron.
μ = [n(n + 2)]1/2
μ = [4×6]1/2
μ = 5BM
Steriochemistry is optically inactive as it is a tetrahedral complex and the relative position of each ligand will be same in all condition.
(v) The complex is anion with manganese as central atom , and six cyanide ions with each unit ngative charge. Balance overall charge
X + 6(-1) = -4
X = + 2
Oxidation state of Mn = + 2
Electronic configuration of Mn:3d3
Name of compound: potassium hexacyanoferrate(III)
Coordination no. Is 6.
As cyanide ions are strong field ligand , they cause pairing of electrons.
No. Of unpaired electrons is 1.
μ = [n(n + 2)]1/2
μ = [1×3]1/2
μ = 1.732BM.
Steriochemistry: optically inactive .