What is meant by stability of a coordination compound in solution? State the factors which govern stability of complexes.
Stability of coordination compounds in a solution refers to the association of the two species i.e coordination sphere and counter ions involved in a state of equilibrium. Stability of complex is expressed in terms of formation constant as:
Stability constant = [ML3]/[M][L]3
The more will be the value of stability constant, the more will be the amount of [ML3] in the solution.
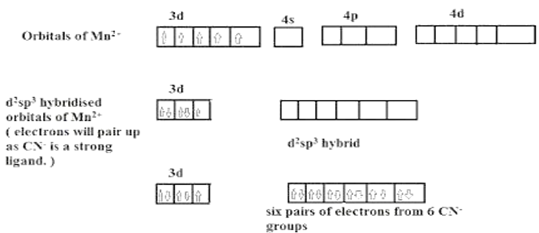
Stability can be of 2 types:
Thermodynamics stability (the extent to which complex is formed or will be transformed to other species at point of equilibrium)
Kinetic stability (speed at which the complex is formed)
Stability of complex depends on factors:
(i) Charge on central atom:. More will be the charge on central atom more will be the strong bond between atom and ligand and more will be the stability of the complex.
(ii) Basic nature of ligand: More will be the basic character of ligand , more it will try to donate the electrons to the metal atom, hence greater stability.
(iii) Presence of chelate ring: The presence of chelate ring give more stability to the complex. (Stronger interaction)