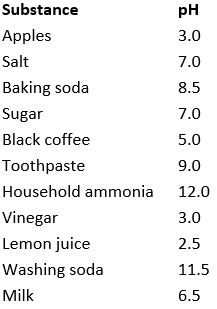
A group of students measured the pH of some substances they found in their homes. Their results are given in the following table:
(a) What would the students have used to measure the pH?
(b) Which solution is the most acidic?
(c) Which solution is the most alkaline?
(d) Which solutions are neutral?
(e) Which solution can be used to treat wasp stings?
(f) Which solution can be used to treat bee stings?
(b) According to the records, Lemon juice with lowest pH of 2.5 is most acidic.
(c) According to results, household ammonia has highest pH indicating it as most alkaline.
(d) Solutions having pH 7 are the neutral solutions such as salt and sugar solutions.
(e) Wasp sting is alkaline in nature. Hence its effect can be neutralized by vinegar(acetic acid), which is acidic in nature.
(f) Baking soda/ sodium hydrogen carbonate is alkaline in nature and it will neutralize the effect of methanoic acid present in bee sting.