Translate the following statements into chemical equations and then balance the equations :
(a) Hydrogen sulphide gas bums in air to give water and sulphur dioxide.
(b) Phosphorus bums in oxygen to give phosphorus pentoxide.
(c) Carbon disulphide bums in air to give carbon dioxide and sulphur dioxide.
(d) Aluminium metal replaces iron from ferric oxide, Fe203, giving aluminium oxide and iron.
(e) Bi:uiurn chloride reacts with zinc sulphate to give zinc chloride and barium sulphate.
(a) 2H2S + 302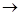 2H20 + 2S02
2H20 + 2S02
(b) P4 + 502![]() 2P205
2P205
(c) CS2 + 302![]() C02+ 2S02
C02+ 2S02
(d) 2Al + Fe203![]() Al203 + 2Fe
Al203 + 2Fe
(e) BaCl2 +ZnS04![]() ZnCl2+ BaS0
ZnCl2+ BaS04
10