(b) Write balanced chemical equation from the following information:
An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
Ca(OH)2 (aq) + C02 (g) 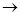 CaC03 (s) + H20 (l)
CaC03 (s) + H20 (l)
This reaction is balanced as same amount of elements and compound is on either side.
20