Compare the stability of +2 oxidation state for the elements of the first transition series.
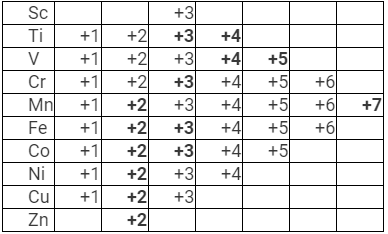
It can be observed from the above table that in the starting of 3d transition series elements like Sc, Ti, V, Cr in +2 state are not that stable in their elements in the +3 state.
In the middle Mn2+, Fe2+, Co2+are quite known. In fact, Mn2+ and Mn7+ are most stable states in Mn. Fe2+ is less stable when compared to Fe3+ which is due to fact that Fe3+ is able to loose one electron to acquire d5 state which has extra stability. Co2+ is less stable as compared to Co3+. Ni2+ is the most common and stable among its +2, +3, +4 states. Cu2+ is more stable and is quite common as compared to Cu+. Towards the end, Zn forms only Zn2+ which is highly stable as it has 3d10 state.
Note: As it becomes difficult to remove the third electron from d-orbital, the stability of +2 oxidation state increases from top to bottom.