What can be inferred from the magnetic moment of the following complex species?
Example. | Magnetic moment |
[K4 [Mn(CN)6] | 2.2 |
[Fe(H2O)6]2+. | 5.3 |
K2 [MnCl4] | 5.9 |
To calculate magnetic moment of the complex species, we use the spin formula:
μ =√n(n+2) BM
When n= 1 | ⇒ μ = √1(1+2) ⇒ u= √3 ⇒ u=1.73 BM |
When n=2 | ⇒ μ = √2(2+2) ⇒ μ = √8 ⇒ μ = 2.83 BM |
When n= 3 | ⇒ μ = √3(3+2) ⇒ μ = 15 ⇒ μ = 3.87 BM |
When n = 4 | ⇒ μ = √ 4(4+2) ⇒ μ = √24 ⇒ μ = 4.899 BM |
When n= 5 | ⇒ μ = √5(5+2) ⇒ μ = √35 ⇒ μ = 5.92 BM |
1. [K4 [Mn(CN)6]
⇒μ = 2.2 BM (given)
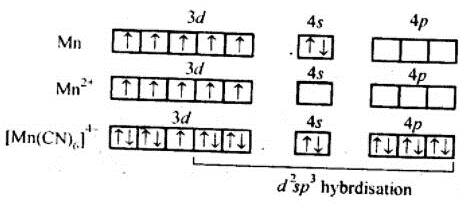
We can see from the above calculation that the given value(2.2) is close to n=1. It means that it has only one unpaired electron Also in this complex Mn is in +2 oxidation state,i.e., as Mn2+. Thus when CN- ligands approach Mn2+ ion, The electrons in 3d do not pair up.
Theatomic number of Manganese (Mn) is Z = 25
The electronic configuration of 25Mn= [Ar] 3d5 4s2
And, the electronic configuration of Mn2+=[Ar] 3d5
Thus CN- is a strong ligand.
The hybridization involved is d2sp3 forming inner orbital octahedral complex
2.[Fe(H2O)6]2+.
⇒ μ = 5.3 BM (given)
We can see from the above calculation that the given value(5.3) is close to n = 4. It means that it has four unpaired electrons. In this complex, Fe is in +2 state, i.e., as Fe2+. This means that the electrons in 3d do not pair up when the ligands, H2O molecules approach.
Theatomic number of Iron(Fe)is Z = 26
The electronic configuration of 26Fe= [Ar] 3d6 4s2
And, the electronic configuration of Fe2+=[Ar] 3d6
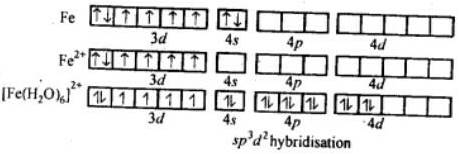
Thus H2O is a weak ligand. To accommodate the electrons donated by six H2O molecules, the hybridization will be sp3d2. Hence it will be an outer orbital octahedral complex.
3. K2 [MnCl4]
⇒μ = 5.9 BM (given)
We can see from the above calculation that the given value(5.9) is close to n=5. It means that it has five unpaired electrons
In this complex, Mn is in +2 state, i.e., as Mn2+. Hence, we can say that Cl- is a weak ligand and does not cause the pairing of electrons.
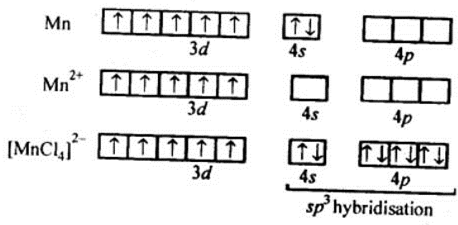
Theatomic number of Manganese (Mn) is Z = 25
The electronic configuration of 25Mn= [Ar] 3d5 4s2
And, the electronic configuration of Mn2+=[Ar] 3d5
Thus, the hybridization involved will be sp3 and the complex will be tetrahedral complex
Note: Being Lewis bases (those who donate electrons) the ligands with less electronegativity will be stronger. Therefore, in general halogen or oxygen donors (F-, Cl-,Br-, H2O) are weak field ligands and the ones in which carbon or nitrogen atom is the donor (eg-CN-,CO,NH3) are strong field ligands.