(a) What is a redox reaction? Explain with an example.
(b) When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidized or reduced? Why?
(c) In the reaction represented by the equation:
![]()
(i) name the substance oxidised.
(ii) name the oxidising agent.
(iii) name the substance reduced.
(iv) name the reducing agent.
(a) Oxidation and reduction happening simultaneously in a reaction is called redox reaction.
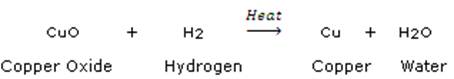
In the above reaction, copper oxide is reduced to copper whereas hydrogen is oxidized to water.
(b)Magnesium is oxidized; because oxygen is added to magnesium creating MgO.
(c) (i) HCl is oxidized.
(ii)MnO2 is reduced.
(iii)MnO2 is oxidizing agent.
(iv) HCl is oxidizing agent.
26