(a) Give an example of an oxidation reaction.
(b) Is oxidation an exothermic or an endothermic reaction?
(c) Explain, by giving an example, how oxidation and reduction proceed side by side.
(a) C (s) + O2 (g) 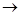 CO2 + Heat
CO2 + Heat
Carbon is oxidized to form carbo dioxide.
(b)Oxidation is an exothermic reaction as heat is given by reaction to the surrounding.
(c) 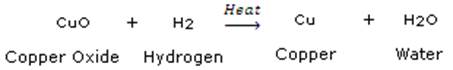
Copper oxide is reduced to copper element and hydrogen gas is oxidized to Dihydrogen oxide(water).
29