What is a decomposition reaction? Give an example of a decomposition reaction. Describe an activity to illustrate such a reaction by heating.
When in a reaction compound splits two or more simpler substance the reaction is called decomposition reaction.
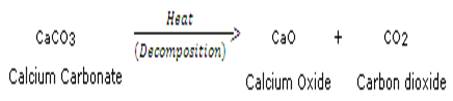
Cacium carbonate decomposes when heated into calcium oxide and carbon dioxide.
Activity: Potassium chlorate decomposes to give Potassium chloride and oxygen, when heated in presence of catalyst manganese dioxide.

Potassium Chlorate a single compound, is splitting up into two simpler substances, potassium Chloride and oxygen. This decomposition reaction is used to prepare oxygen gas in laboratory.
30