When a magnesium ribbon is heated, it burns in air to form magnesium oxide. Write a balanced chemical equation for this reaction. Name (i) substance oxidised, and (ii) substance reduced.
2Mg + O2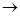 2Mg0
2Mg0
(i) Mg is oxidized
(ii) O2 is reduced.
35
(i) Mg is oxidized
(ii) O2 is reduced.