Why are decomposition reactions called the opposite of combination reactions? Explain with equations of these reactions.
In decomposition reaction one compound decomposes to give two simpler compound or element whereas in combination reaction two different compound or element fuses or combine to form one single compound.
Ex- Decomposition Reaction:
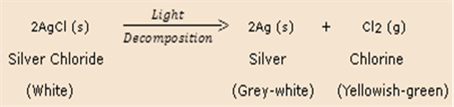
Here Agcl(s) decomposes to give Ag(s) and Cl(s)
Combination Reaction:
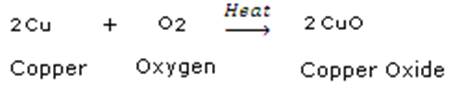
Here, Copper and Oxygen combine to give Copper Oxide.
37