Calculate and compare the energy released by a) fusion of 1.0 kg of hydrogen deep within Sun and b) the fission of 1.0 kg of 235U in a fission reactor.
a) Mass of hydrogen, m = 1kg = 1000 g
Since,1 mole of hydrogen contains 6.023×1023 atoms which is equivalent to 1 g of hydrogen then, 1kg of hydrogen contains,
N = 6.023×1023×1000 = 6.023×1026 atoms
In sun, 4 hydrogen atoms, ![]() combine to form one helium atom,
combine to form one helium atom, ![]() in fusion process which releases 26 MeV of energy.
in fusion process which releases 26 MeV of energy.
Thus,
Energy released from fusion of 1kg of hydrogen is,
![]() ……………….(1)
……………….(1)
= ![]()
b) Mass of uranium, m = 1kg = 1000 g
Since,1 mole of Uranium contains 6.023×1023 atoms which is equivalent to 235 g of Uranium then, 1kg of Uranium contains,
N = ![]()
During fission reaction of 1 atom of ![]() releases 200 MeV of energy.
releases 200 MeV of energy.
Thus,
Energy released from fission of 1kg of Uranium is,
![]() ………..(2)
………..(2)
Divide (1) by (2) we get,
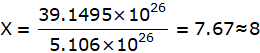
Hence, Fusion reaction occurred in Sun releases 8 times more energy than energy released during the fission reaction of Uranium.