The following diagram shows a part of the periodic table in which the elements are arranged according to their atomic numbers. (The letters given here are not the chemical symbols of the elements):
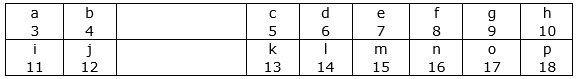
(i) Which element has a bigger atom, a or f?
(ii) Which element has a higher valency, k or o?
(iii) Which element is more metallic, i or k?
(iv) Which element is more non-metallic, d or g?
(v) Select a letter which represents a metal of valency 2.
(vi) Select a letter which represents a non-metal of valency 2.
(i) an element has a bigger atom because when we move left to right the size of the atom decreases.
(ii)The valency of o element is 1 and k element is 3.
(iii) When we move from left to right the metallic character decreases, so element i is more metallic.
(iv) When we move left to right in the period then the non- metallic character increases, so element g is more non metallic.
(v) letter b represents a metal of valency 2.
(vi) letter f represents a non metal of valency 2.