The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters a, b, c, d and e (which are not their chemical symbols):
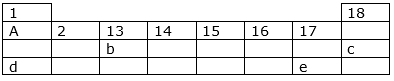
(i)Select the letter which representsan alkali metal.
(ii) Select the letter which represents a noble gas.
(iii) Select the letter which represents a halogen.
(iv) What type of bond is formed between a and e?
(v) What type of bond is formed between d and e?
(i) the letter d which representsan alkali metal
(ii) the letter c which represents a noble gas
(iii) the letter e which represents a halogen
(iv) The bond formed between a and e is a covalent bond.
(v) The bond formed between d and e is ionic bond.
74