Identify the substances that are oxidised and the substances that are reduced in the following reactions:
(i) 4Na (s) + O2 (g) → 2Na20 (s)
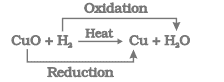
(ii) CuO (s) + H2 (g) → Cu (s) + H2O (l)
(i)
![]()
If a substance gain oxygen during a reaction, it is said to be oxidized. During this reaction, the sodium is gaining oxygen and is being oxidized. Oxygen is getting reduced.
(ii) ![]()
During this reaction, copper oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidized.
35