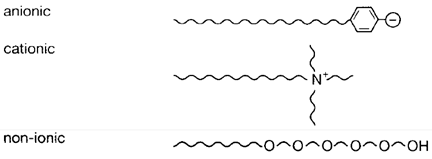
Explain the following terms with suitable examples
(i) cationic detergents
(ii) anionic detergents
(iii) non-ionic detergents
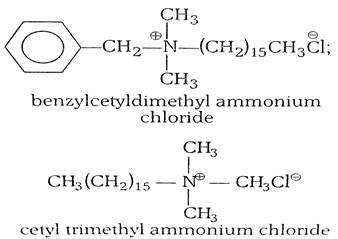
(i) cationic detergents- Cationic detergents are quaternary ammonium salts [chlorides, bromides, acetates, etc.] having long chain alkyl groups. The cationic detergents are more expensive than anionic detergents and hence they find only limited use. However, they possess germicidal properties and are used quite extensively as germicides. The examples of cationic detergents are as follows:
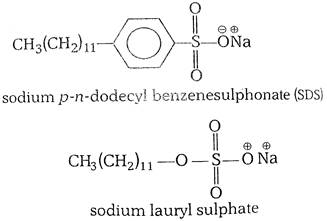
(ii) anionic detergents- A detergent is said to be anionic when the large part of its molecule is an anion and is involved in the cleansing action. The anionic detergents are also effective in slightly acidic solutions. In slightly acidic solutions, they form an alkyl hydrogen sulphate which is a soluble material. In contrast, soaps react with acidic solutions to form insoluble fatty acids.The examples of anionic detergents are as follows:
(iii) non-ionic detergents- These do not contain any ion in their constitution. They are the esters of high molecular mass formed by the reaction between polyethylene glycol and stearic acid. Some non-ionic detergents are used as dishwashing detergents.The example of a non-ionic detergent is as follows: