Arrange the following compounds in increasing order of their reactivity in nucleophilic addition reactions.
Benzaldehyde, p-Tolualdehyde, p-Nitrobenzaldehyde, Acetophenone.
Hint: Consider steric effect and electronic effect.
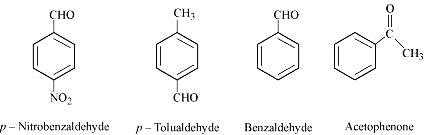
The +I effect is more in ketone than in aldehyde because in ketone there are 2 alkyl groups contributing in the +I effect whereas in aldehydes there is only one alkyl group. Hence, acetophenone(being ketone group attached to it) is the least reactive in nucleophilic addition reactions. Among aldehydes, the +I effect is the highest in p-tolualdehyde because of the presence of the electron-donating –CH3 group(which increase the electron density on the carbonyl carbon via resonance through the benzene ring) and the lowest in p-nitrobenzaldehyde because of the presence of the electron-withdrawing –NO2 group(which decreases the electron density on the carbonyl carbon via resonance through the benzene ring). Hence, the increasing order of the reactivities of the given compounds is:
Acetophenone<p-tolualdehyde<Benzaldehyde<p-Nitrobenzaldehyde