An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen.
The molecular mass of the compound is 86. It does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogensulphite and give positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acid. Write the possible structure of the compound.
C = 69.77% ie =![]() =5.88;
=5.88;
H=11.63% ie=![]() =11.63;
=11.63;
O=(100-(69.77+11.63))=18.6% ie=![]() =1.16
=1.16
Molecular Ratio will be 5.88:11.63:1.16=5:10:1.
Empirical formula is C5H10O, molecular weight will be ![]() =86.
=86.
Hence molecular formula will be same.
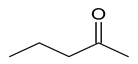
It does not reduce tollen’s reagent, it is not an aldehyde. The compound is addition product of sodium hydrogen sulphite it must be a ketone, It is giving positive iodoform test it is ethyl ketone. On vigorous oxidation, it forms ethanoic acid and propanoic acid the given compound is pentane-2-one.