Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?
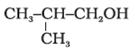
In the Grignard reagent reaction, the first step of the reaction is the nucleophilic addition of Grignard reagent to the carbonyl group to form an adduct, Hydrolysis of adduct results in the formation of alcohol.
Here, is the general reaction with Grignard reagent below:-
![]()
From here, it is clear that HCHO gives CH2OH groups, so R of Grignard reagent is the remaining part of given alcohols. Thus, select the suitable Grignard reagent by substituting the value of R. Now we can see the reaction given below:-
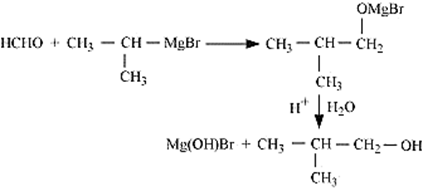
Methanal reacts with iso-propyl magnesium bromide, in presence of dry ether gives an additional compound. And this additional compound on reaction with H2O /H+ gives iso-butyl alcohol (i.e., 2-methylpropane-1-ol) as one of the final product.