Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Here, propanol undergoes intermolecular H-bonding because of the presence of -OH group while butane has no such property.
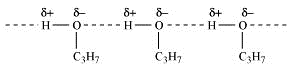
(intermolecular Hydrogen bonding in propanol)
Therefore, extra energy will be required to break those hydrogen bonds which in turn causes higher boiling point for propanol when compared to butane.
6