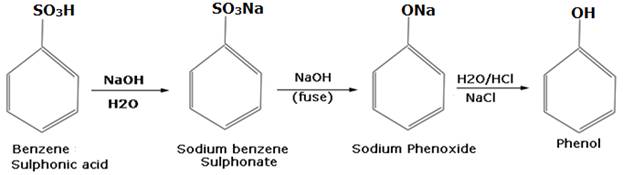
You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
The reaction given below is:
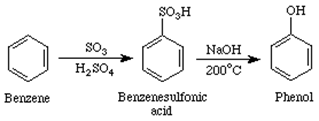
Benzene reacts with concern. H2SO4 and undergoes the following mechanism:-
Step 1: The equilibrium produces SO3 in concentrated H2SO4, as shown below:
![]()
Step 2: SO3 is the electrophile which reacts with benzene to form arenium ion, as shown below:
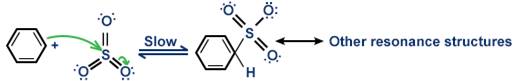
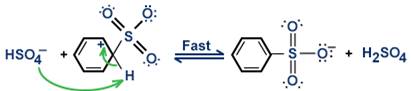
Step 3: A proton is removed from the arenium ion to form benzenesulfonate ion.
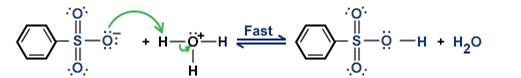
Step 4: The benzenesulfonate ion accepts a proton to become benzene-sulphonic acid, as shown below:
Step 5: The benzene sulphonic acid then reacts with NaOH to give phenol as the final product, as shown below:
14