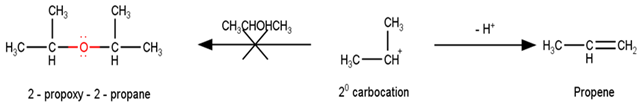
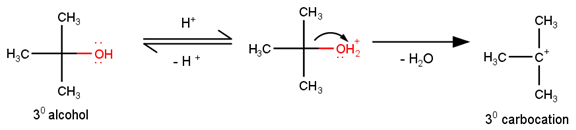
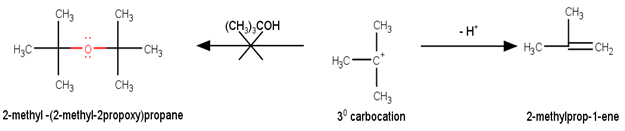
Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
The preparation of ether by acid dehydration of primary alcohol involves the nucleophilic addition of alcohol molecule to the protonated alcohol molecule as shown below: -
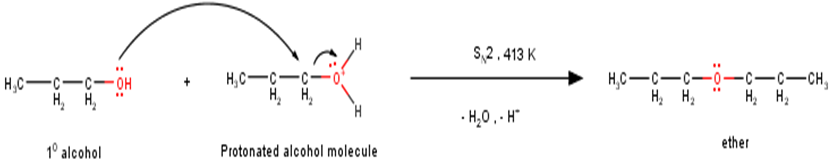
However, under these conditions secondary and tertiary alcohols forms alkenes rather than ethers. The reason for this being that due to stearic hindrance, nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not take place. Instead protonated 20 and 30 alcohols lose a molecule of water to form stable carbocations. The stable carbocations so formed prefers to lose a proton to form alkenes instead of forming ethers by undergoing nucleophilic attack by another alcohol molecule. This could be seen clearly from the following reactions : -