Account for the following:
Although amino group is o– and p– directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m-nitroaniline.
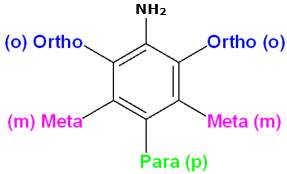
The below diagram shows the position of ortho(o), para(p) and meta(m) derivatives of amino group:
Electrophilic addition reaction of amines: In addition to the reaction of the amino group (NH2 group), aromatic amines also undergo typical electrophilic substitution reactions of the aromatic ring. In all these reactions, the NH2 group strongly activates the aromatic ring through delocalization of the lone pair of electrons of the N-atom over the aromatic ring.

As a result, electron density increases more at ortho and para positions. Therefore NH2 group directs the incoming group to ortho and para positions, i.e., NH2 is an o-, p-directing group.
But it has been observed that on nitration of aniline gives a substantial amount of m-nitroanilne.
Explanation: Nitration is usually carried out in acidic medium in the presence of concentrated HNO3 and concentrated H2SO4. As a result, most of the aniline is converted into anilinium ion(gets protonated) and since - +NH3 is m-directing group, therefore, an unexpected large amount of m-nitroaniline is obtained.
Nitration of anilne gives mainly p-nitroaniline

Nitration of anilinium ions give m-nitroanilne(due to protonation)
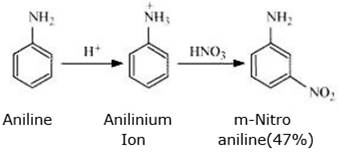
Hence, nitration of aniline gives a mixture of p-nitroaniline and m-nitroaniline in approx. 1:1 ratio.