Account for the following:
Gabriel phthalimide synthesis is preferred for synthesising primary amines.
Gabriel phthalimide synthesis is a very convenient method for the preparation of pure aliphatic amines ( especially primary amines) Phthalimide on treatment with ethanolic KOH gives potassium phthalimide which on heating with a suitable alkyl hallide gives N-substituted phthalimides. These upon susbsequent hydrolysis with dil.HCl under pressure or with alkali gives primary amines.
Step 1: Phthalimide is treated with KOH to form potassium phthalimide
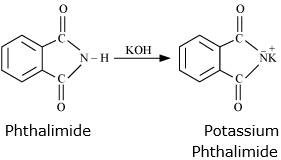
Step 2: Potassium phthalimde is treated with suitable alkyl hallide to form N-substituted phthalimides.
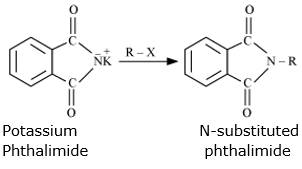
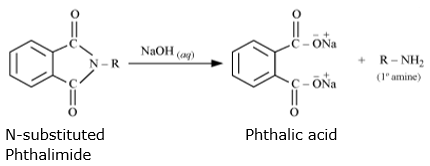
Step 3: N-substituted phthalimides undergoes hydrolysis in the prsence of dil HCl or with alkali(NaOH) to give primary amines.
Overall reaction:
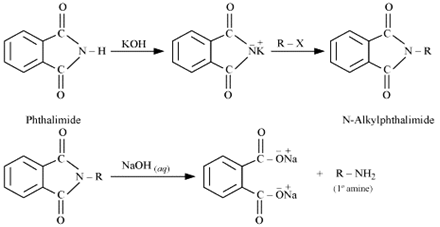
∴ Gabriel phthalimide synthesis results in the formation of primary(1° amine) only. Secondary or tertiary amines are not formed through this synthesis. Hence, Gabriel phthalimide synthesis preferred for the formation of primary amines only.