Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
(i) ![]()
(ii) 
(iii) ![]()
In SN2 Reaction, the product will be formed in a single step with no intermediates. Nucleophile attack will be easier for simple halides than hindered haloalkanes. Therefore,
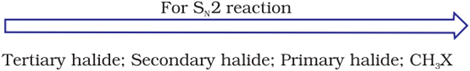
Because of this in SN2
i) Bromobutane (1°)reacts faster than 2-Bromobutane(2°)
ii) 2-Bromobutane(2°) reacts faster than 2-Bromo-2-methylpropane or tert-Butyl bromide (3°)
iii) 1-Bromo-3-methyl butane reacts faster than the 1-Bromo-2-methyl butane as the former is less hindered with respect to leaving halide than the later which is more hindered comparatively.
12