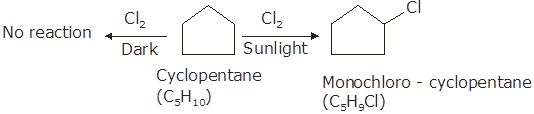
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
The hydrocarbon with molecular formula C5H10 can either form a cycloalkane or an alkene. Since the compound does not react with Cl2in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.
13