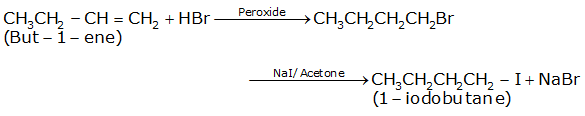
Write the equations for the preparation of 1-iodobutane from
but-1-ene.
The conversion of but-1-ene to 1-iodobutane takes place according to anti-markovnikoff (positive charged ion H+ goes to the carbon which has less number of hydrogens and negative part Br- goes to the carbon which has more number of hydrogens.) i.e. the attack of HBr on but-1-ene in the presence of peroxide gives 1-bromobutane as the intermediate which on treatment with NaI+Acetone gives 1-iodobutane as the final product and NaBr as the by-product.
14