The unit of length convenient on the atomic scale is known as an angstrom and is denoted by Å: 1 Å = 10–10 m. The size of a hydrogen atom is about 0.5 Å. What is the total atomic volume in m3 of a mole of hydrogen atoms?
Given
Radius of hydrogen atom, r = 0.5 Å = 0.5 × 10-10 m
Volume of hydrogen atom, V = (4/3)πr3
⇒ 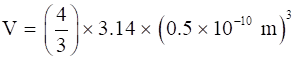
⇒ V = 5.24 × 10-31 m3
One mole of hydrogen atom contains NA = 6.023 × 1023 atoms.
Where, NA is Avogadro’s number.
So, Volume of one mole of hydrogen atom,
Vt = 6.023 × 1023 × 5.24 × 10-31 m3
⇒ Vt = 3.16 × 10-7 m3
24