Explain why
The angle of contact of mercury with glass is obtuse, while that of water with glass is acute.
The angle between the glass surface and tangent to the liquid surface at the point of contact with the glass surface is known as angle of contact (θ)
![]()
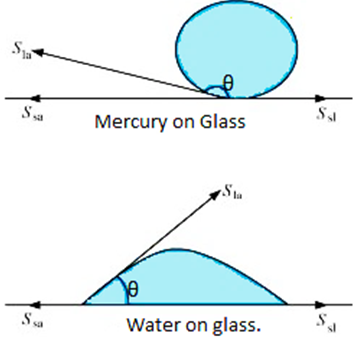
Figure showing various tensions at the interfaces
Let Sla = Interfacial tension between liquid-air
Ssa = Interfacial tension between solid-air
Ssl = Interfacial tension between solid-liquid
At the line of contact, the surface forces between the three media should be in equilibrium.
SlaCosθ = Ssa - Ssl
cosθ = (Ssa - Ssl)/Sla
We also know that surface force is directly proportional to the density of the substance.
In case of mercury, the density of mercury is greater than that of density of glass, therefore cohesive forces exerted by mercury molecules are greater than adhesive forces between the glass and the mercury molecules .But density of air is less that of density of glass; so adhesive forces are greater than the cohesive forces. The surface tension of a liquid results from the imbalance of the cohesive forces between molecules. Therefore interfacial tension between the glass and mercury is greater than the tension between solid-air surface.
So, Sla>Ssa.
cosθ = (Ssa - Ssl)/Sla is negative. Hence θ is an obtuse.
In case of water, the density of water is lesser than that of density of glass molecules, therefore cohesive forces exerted by water are lesser than adhesive forces between the glass and water molecules. Therefore the interfacial tension between glass-water is less than tension between air-glass surface.
So, Sla<Ssa
cosθ = (Ssa - Ssl)/Sla is positive. Hence θ is acute.