From a certain apparatus, the diffusion rate of hydrogen has an average value of 28.7 cm3 s–1. The diffusion of another gas under the same conditions is measured to have an average rate of 7.2 cm3 s–1. Identify the gas.
The Graham’s Law of Diffusion states that the rate of diffusion or of effusion of a gas is inversely proportional to the square root of its molecular weight. We have,
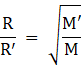
Where,
M is the molecular mass of the first gas,
M’ is the molecular mass of the second gas
R is the diffusion/ effusion rate of the first gas,
R’ is the diffusion/ effusion rate of the second gas
Given,
Molecular mass of hydrogen, M = 2.020 g
Rate of diffusion of hydrogen, R = 28.7 cm3 /s
Rate of diffusion of unknown gas, R’ = 7.2 cm3 /s
Thus, From the Graham’s law of diffusion, we get:
⇒ ![]()
⇒ 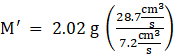
⇒ M’ = 32.09 g
32 g is the molecular mass of Oxygen .
The unknown gas is identified to be Oxygen.