At 0°C, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Density (d) of the substance at temperature (T) can be given by the expression,
⇒ 
Now, density of oxide (d1) is given by,
⇒ 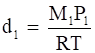
Where, M1 and P1 are mass and pressure of the oxide respectively.
Density of dinitrogen gas (d2) is given by,
⇒ d2 = 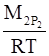
Where,M2 and P2 are the mass and pressure of the oxide respectively.
According to the given question,
⇒ d1 = d2
Molecular mass of nitrogen, M2 = 28 g/mol
⇒ M1P1 = M2P2
Therefore, we can write
Hence molar mass
⇒ 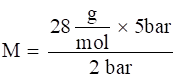
⇒ M = 70 g/mol
Hence, the molecular mass of the oxide is 70 g/mol
5