Write the expression for the equilibrium constant, Kc for each of the following reactions:
(i) 2NOCl (g) ⇌2NO (g) + Cl2 (g)
(ii) 2Cu(NO3)2 (s) ⇌2CuO (s)+4NO2 (g)+O2 (g)
(iii) CH3COOC2H5(aq) + H2O(l) ⇌CH3COOH (aq) + C2H5OH (aq)
(iv) Fe3+ (aq) + 3OH– (aq) ⇌Fe(OH)3 (s)
(v) I2 (s) + 5F2⇌2IF5
For a chemical change,
aA + bB ⇌ cC + dD
Equilibrium constant Kc for the reaction,
Kc =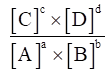
The concentration of solid substance and pure substance are 1.
(i) Kc = 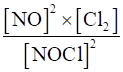
(ii) Kc =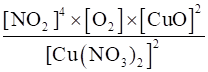
= [NO2]4 [O2]
(iii) Kc =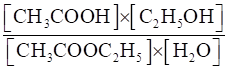
= 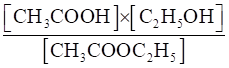
(iv) Kc =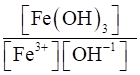
= 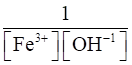
(v) Kc =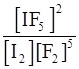
=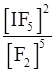
7