Kp= 0.04 atm at 899 K for the equilibrium shown below. What is the equilibrium concentration of C2H6 when it is placed in a flask at 4.0 atm pressure and allowed to come to equilibrium?
C2H6 (g) ⇌ C2H4 (g) + H2 (g)
For the given reaction, lets assume that p atm is the pressure exerted by ethene and hydrogen gas at equilibrium.
Therefore, the given reaction is:
C2H6 (g) ⇌ C2H4 (g) + H2 (g)
![]()
Equilibrium pressure constant (Kp) is defined as a number that expresses the relationship between the partial pressures of products and reactants present at equilibrium in a reversible chemical reaction at a given temperature.
Here. Kp= 0.04 atm
For the given reaction,
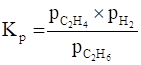
Where, ![]() = partial pressure pf C2H4 in atom at equilibrium
= partial pressure pf C2H4 in atom at equilibrium
![]() = partial pressure of H2 in atom at equilibrium
= partial pressure of H2 in atom at equilibrium
![]() = partial pressure of C2H6 in atom at equilibrium
= partial pressure of C2H6 in atom at equilibrium
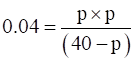
p2=0.16-0.04p
p2+0.04p-0.16=0
Now, 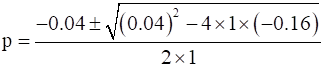
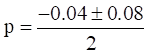
 (Taking positive value)
(Taking positive value)
p=0.38
Therefore at equilibrium, [C2H6]= 4 - p = 4 - 0.38
=3.62 atom