Predict which of the following reaction will have appreciable concentration of reactants and products:
A. Cl2 (g) ⇌ 2Cl (g) Kc= 5 ×10–39
B. Cl2 (g) + 2NO (g) ⇌ 2NOCl (g) Kc = 3.7 × 108
C. Cl2 (g) + 2NO2 (g) ⇌ 2NO2Cl (g) Kc = 1.8
The reaction given in (c) will have appreciable concentration of
reactants and products whose Kc are 1.8.
Explanation:
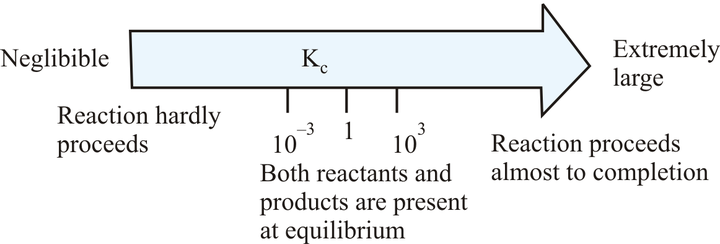
If the value of Kc lies between 1 × 10-3 and 1 × 103, a reaction has an appreciable concentration of reactants and products.
⇒In reaction (A),
Cl2 (g) ⇌ 2Cl (g)
Kc= 5 ×10–39 (given)
The value of Kc does not lie between 1× 10-3 and 1× 103 Hence, this reaction has not the appreciable concentration of reactants and products.
⇒In reaction (B),
Cl2 (g) + 2NO (g) ⇌ 2NOCl (g)
Kc = 3.7 × 108 (given)
The value of Kc does not lie between 1× 10-3 and 1×103 Hence, this reaction has not appreciable concentration of reactants and products.
⇒In reaction (B),
Cl2 (g) + 2NO2 (g) ⇌ 2NO2Cl (g)
Kc = 1.8 (given)
The value of Kc lies between 1× 10-3 and 1×103.Hence, this reaction has appreciable concentration of reactants and products.
Note: If Kc > 103, products predominate over reactants, i.e., if Kc is very large, the reaction proceeds nearly to completion.
If Kc < 10–3, reactants predominate over products, i.e., if Kc is very small, the reaction proceeds rarely.