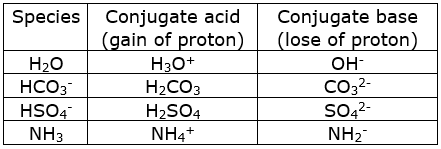
The species: ![]() can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
Bronsted acid: A bronsted acid is a substance that can donate a proton. A bronsted acid is proton-donor.
Bronsted base: A bronsted base is a substance that can accept a proton from an acid. A bronsted base is proton-acceptor.
When a base gain a proton, the residual part of it has a tendency to accept the proton. Therefore, it behaves as conjugate acid.
When an acid loses a proton, the residual part of it has a tendency to regain a proton. Therefore, it behaves as a conjugate base.
Conjugate base ⇌ Conjugate acid - H+
Conjugate base ⇌ Conjugate acid + H+
42