Calculate the degree of ionization of 0.05M acetic acid if its pKa value is 4.74. How is the degree of dissociation affected when its solution also contains (a) 0.01M (b) 0.1M in HCl?
Given:
Concentration of acetic acid = 0.05M
pKa = 4.74
As we know that, pka = -logKa
We can also write,
∴ pKa = -logKa
⇒4.74 = -logKa
By taking antilog of both the sides, we get
Antilog -4.74 = Ka
⇒Ka = 1.82 × 10-5
By applying the formula, ka = cα2
Where c is the concentration and α is the degree of ionization
We can also write,
α =√ 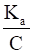
Ka = 1.82 × 10-5(given)
C = 0.05M (given)
∴ α =√ 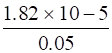
⇒α = 1.908 × 10-2
In the presence of HCl due to the high concentration of H+ , the dissociation equilibrium will shift in the backward direction i.e., dissociation of acetic acid will decrease.
a) In the presence of HCl, let x be the amount of acetic acid dissociated after the addition of HCl.
Ionization of acetic acid
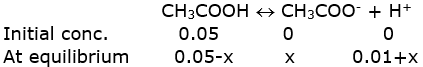
We can assume, 0.05-x≈ 0.05
0.01+x ≈ 0.01
To calculate the degree of ionization, we apply the formula
α=
Where α = 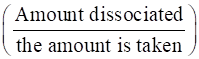
Ka is the ionization constant
c is the concentration
Ka = 1.82 × 10-5(given)
C = 0.01M (given)
∴ α = 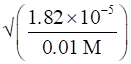
⇒α =1.82 × 10-3
Thus, the degree of ionization in the presence of 0.01M HCl is 1.82 × 10-3
b) In the presence of 0.1 M HCl, let y be the amount of acetic acid dissociated after the addition of HCl.
Ionization of acetic acid
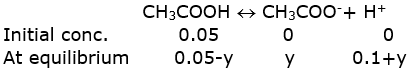
We can assume, 0.05-x≈ 0.05
0.1+x ≈ 0.1
To calculate the degree of ionization, we apply the formula
α=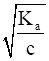
Where α = 
Ka is the ionization constant
c is the concentration
Ka = 1.82 × 10-5(given)
C = 0.1M (given)
∴ α = 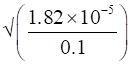
α =1.82 × 10-4
Thus, the degree of ionization in the presence of 0.1M HCl is 1.82 × 10-4