The ionization constant of dimethylamine is 5.4 × 10–4. Calculate its degree of ionization in its 0.02M solution. What percentage of dimethylamine is ionized if the solution is also 0.1M in NaOH?
Given:
Kb = 5.4 × 10–4.
c=0.02M
To calculate the degree of ionization, we apply the formula
α=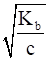
Where Kb is the ionization constant
c is the concentration
∴ α =√ 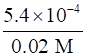
⇒α =0.1643
Thus, the degree of ionization is 0.1643
Ionization of 0.1 M NaOH;
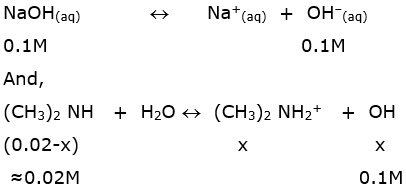
Since, (CH3)2 NH+2]=x
[OH– ]=x+0.1 ≈ 0.1 M
Using the formula,
Kb = 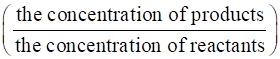
⇒Kb = 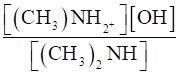
As Kb = 5.4 × 10–4.(given)
[(CH3)2 NH] = 0.02 M (given)
∴ 5.4 × 10–4 = 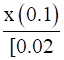
⇒x = 0.054
Thus, It means that in the presence of 0.1 M NaOH, 0.54% of dimethylamine will get dissociated.
55