If 0.561 g of KOH is dissolved in water to give 200 mL of solution at 298 K.
Calculate the concentrations of potassium, hydrogen and hydroxyl ions. What is its pH?
Given:
Mass of KOH (m) = 0.561g
Volume(V) = 200 ml =  L = 0.2L
L = 0.2L
Molar mass of KOH (M) = 56 g/mol
To calculate concentration of KOH, we apply the formula,
Concentration= 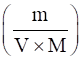
⇒[KOH] = 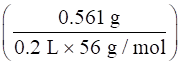
⇒[KOH] = 0.05 M
Now, the ionisation of KOH
⇒KOH↔ K + OH-
At equilibrium
∴ [K] = [OH-] = 0.05 M
To calculate [H+], we apply the formula:
Kw = [H+] [OH-]
Where Kw is the ionic product of water which is defined as the product of molar concentration of H+ and OH- ions.
Using the formula, we write
[H+] = 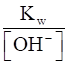
As the value of Kw is taken as 10-14
[OH-]= 0.05 M (calculated)
∴ [H+] = 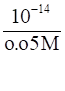
⇒[H+] = 2.0 × 10-13
Thus, the concentration of potassium, hydrogen, and hydroxyl ions are 0.05, 0.05 and 2.0 × 10-13 respectively
To calculate pH, we apply the formula:
pH = –log [H+]
⇒pH = - log (2.0 × 10-13)
⇒pH = 13- log2
⇒pH = 12.69
Thus, the pH is 12.69