What are the oxidation number of the underlined elements in each of the following and how do you rationalise your results?
CH3COOH
C2H4O2
2(x) + 4(+ 1) + 2(-2) = 0
2x + 4-4 = 0
X = 0
However,0 is average O.N. of C. The two carbon atoms present in this molecule are present in the different environments. Hence, they cannot have the same oxidation number.
Thus, C exhibits the oxidation states of + 2 and -2 in CH3COOH.
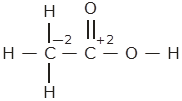
5