What is the relationship between the members of following pairs of structures?
Are they structural or geometrical isomers or resonance contributors?

Compounds having the same molecular formula, the same constituition , and the same sequence of covalent bonds,but with different relative position of their atoms in space are called geometrical isomers.
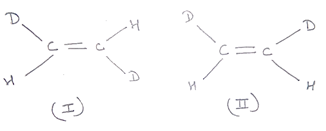
In structures 1 and 2, the relative position of Deuterium (D) and hydrogen(H) in space are different. Hence, the given pairs represent geometrical isomers.
16