Define the bond length.
The bond length is defined as the average distance between the nuclei of two bonded atoms in a molecule.
Example:
Let us consider a diatomic molecule. The atoms in this molecule are always vibrating with respect to each other. The question of any fixed distance between them, therefore, does not arise. We can, however, think of an average distance between the nuclei of the two atoms bonded to each other. This is called bond length or bond distance.
Thus,
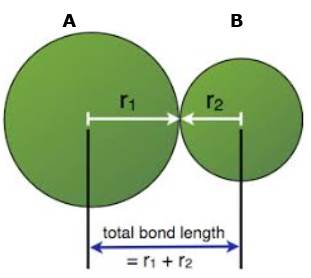
The bond length in a covalent molecule AB.
R= r1 + r2
Where R is the bond length
R1 and r2 are the covalent radii of atoms A and B respectively
Note: The bond length decreases with a multiplicity of the bond formed between the two atoms. Thus, the C Ξ C bond is shorter than C=C bond which in turn, is shorter than C—C bond.