The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid.

The correct structure of CH3COOH is:
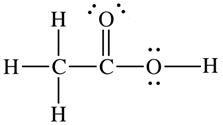
Explanation:
The atomic number of C is 6 and no. of valence electrons is 4.
The atomic number of O is 8 and no. of valence electrons is 6.
The atomic number of H is 1 and no. of valence electron is 1.
Hence, the total number of valence electrons in the element is:
Two carbons (2× 4 valence electrons) + two oxygen (2× 6 valence electrons) + 4 hydrogen (4× 1 valence electron) = 24 total valence electrons. Hence, the skeletal structure of CH3COOH is:
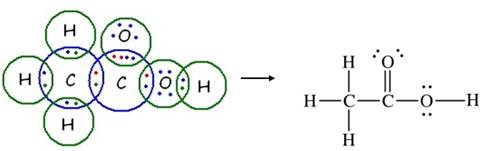
24