Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
Here consider the electronic configuration of carbon atom:
6C: 1s2 2s2 2p2
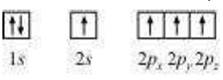
Whereas in the excited stage the orbital diagram can be,
Therefore, the carbon atom undergoes sp3 hybridisation in CH4 and takes the tetrahedral shape.
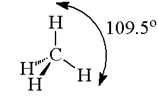
But for a square planar shape, the hybridization of the central atom should be dsp2 Since the atom of carbon does not have any d-orbitals to undergo hybridisation, the structure of CH4 cannot be square planar. Also, with a bond angle of 90° in square planar, the stability of CH4 will be very less because of the repulsion between the bond pairs. Hence, VSEPR theory also favours tetrahedral structure for CH4.