Is there any change in the hybridization of B and N atoms as a result of the following reaction?
BF3 + NH3→ F3B.NH3
Boron atom in BF3 is sp2 hybridized. The excited state of boron can be represented by:
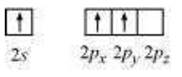
While the nitrogen atom in NH3 is sp3 hybridized. The excited state can be shown as:
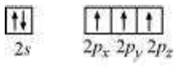
On completion of this reaction, an adduct F3B.NH3 is formed as the hybridization of Boron changes to sp3, while the hybridization of nitrogen remains intact.
31